THE RATIONAL DESIGN OF MINIMUM ESSENTIAL MIXTURES
Our Goal is to preserve the efficacy of plant-based medicines while reducing their complexity for standardization. Our Strategy is to combine our proprietary PhAROS Drug Discovery Platform and High Throughput Screening of Cell and Animal Models of Specific Disease Processes to Create Rationally-Designed, Minimum Essential Mixtures.


PhAROSTM: Phytomedical Analytics for Research Optimization at Scale
- A Science Gateway and Virtual Research Environment for Drug Discovery.
- Provides transformative data integration, analytic methods, and visualization tools for the meta-analysis of non-Western medical knowledge systems.
- Helps pre-validate the efficacy of drug-target-indication relationships.
- Multiple Patent Applications filed
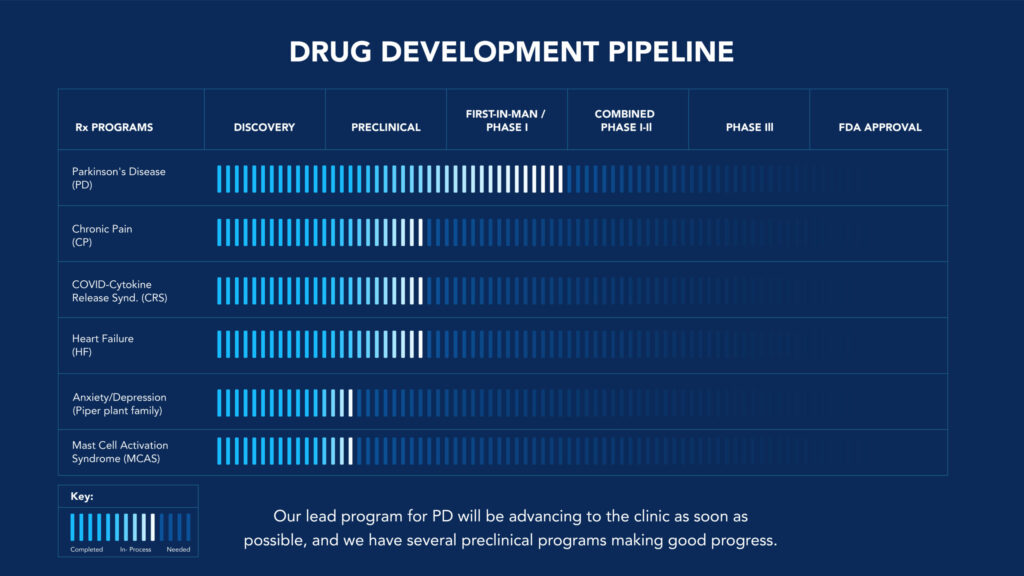

PARKINSON’S DISEASE THERAPIES MEM.PD119, MEM.PD205, and MEM.PD361
- Animal Proof-of-Concept Achieved (April 6, 2020)
- US Patent Issued (May 19, 2020)
- Mechanism of Action Study (on-going at the NRC Canada)
- Investigational New Drug (IND)-Application Preparation
PAIN THERAPEUTICS-GBS.CP110, GBS.CP121, and GBS.CP139
Oral nanoparticles containing our pain formulas.
- US Patent Issued (July 14, 2020)
- Animal Proof-of-Concept Study (on-going at the NRC Canada)
- Scale Up of Oral Nanoparticle Production
CYTOKINE RELEASE SYNDROME THERAPIES for COVID-19 PATIENTS
- US nonprovisional patent filed (August 18th, 2021)
- Positive proof of concept results (at MSU)
- Coronavirus Treatment Acceleration Program (CTAP) for US FDA fast-tracking
Scientific Advisory Board

Dr. Andrea Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Offer and Director, Gb Sciences/GbS Global Biopharma, Inc.

Dr. Helen Turner
VP Innovation, Professor of Natural Sciences & Mathematics at Chaminade University

Dr. Zoltan Mari
Director of the PD and Movement Disorders Program and The Ruvo Family Chair, Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, NV

Dr. Alexander Stokes
Assoc. Professor, Univ. of Hawaii-John A Burns School of Medicine, Center for Cardiovascular Research
Dr. Andrea L. Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Officer and Director of GB Sciences/GBS Global Biopharma, Inc.
Dr. Andrea Small-Howard has more than 20 years of scientific research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. Dr. Small-Howard has taken novel biological products from ideation through commercialization.
Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration (“US FDA”) and numerous international regulatory agencies. Currently, Dr. Andrea Small-Howard leverages her broad biopharmaceutical industry knowledge as the Chief Science Officer and member of the Board of Directors at both GB Sciences, Inc. (OTCQB:GBLX), and their wholly-owned Canadian subsidiary, GBS Global Biopharma, Inc. Dr. Small-Howard brings her passion for advancing research on phytochemical compounds to her current roles.
She envisages bringing more phytomedicines to market through an innovative AI-based drug discovery engine and biopharmaceutical drug development program for disease-specific indications. Dr. Small-Howard has worked on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes.
More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ plant chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s Disease (PD), co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for plant-derived therapeutic compounds.
Dr. Small-Howard is the architect of a strategic vision at GB Sciences/GBS Global Biopharma to make safe, effective, standardized cannabinoid medicines available to patients where its use can be supported with rigorous evidence. To achieve standardization in their cannabinoid-containing, optimized therapeutic mixtures, the Company is using synthetic cannabinoids produced under current Good Manufacturing Practices (cGMP), which are identical in both structure and function to the homologous plant cannabinoids

Dr. Andrea L. Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Officer and Director of GB Sciences/GBS Global Biopharma, Inc.
Dr. Andrea Small-Howard has more than 20 years of scientific research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. Dr. Small-Howard has taken novel biological products from ideation through commercialization.
Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration (“US FDA”) and numerous international regulatory agencies. Currently, Dr. Andrea Small-Howard leverages her broad biopharmaceutical industry knowledge as the Chief Science Officer and member of the Board of Directors at both GB Sciences, Inc. (OTCQB:GBLX), and their wholly-owned Canadian subsidiary, GBS Global Biopharma, Inc. Dr. Small-Howard brings her passion for advancing research on phytochemical compounds to her current roles.
She envisages bringing more phytomedicines to market through an innovative AI-based drug discovery engine and biopharmaceutical drug development program for disease-specific indications. Dr. Small-Howard has worked on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes.
More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ plant chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s Disease (PD), co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for plant-derived therapeutic compounds.
Dr. Small-Howard is the architect of a strategic vision at GB Sciences/GBS Global Biopharma to make safe, effective, standardized cannabinoid medicines available to patients where its use can be supported with rigorous evidence. To achieve standardization in their cannabinoid-containing, optimized therapeutic mixtures, the Company is using synthetic cannabinoids produced under current Good Manufacturing Practices (cGMP), which are identical in both structure and function to the homologous plant cannabinoids

Dr. Andrea L. Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Officer and Director of GB Sciences/GBS Global Biopharma, Inc.
Dr. Andrea Small-Howard has more than 20 years of scientific research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. Dr. Small-Howard has taken novel biological products from ideation through commercialization.
Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration (“US FDA”) and numerous international regulatory agencies. Currently, Dr. Andrea Small-Howard leverages her broad biopharmaceutical industry knowledge as the Chief Science Officer and member of the Board of Directors at both GB Sciences, Inc. (OTCQB:GBLX), and their wholly-owned Canadian subsidiary, GBS Global Biopharma, Inc. Dr. Small-Howard brings her passion for advancing research on phytochemical compounds to her current roles.
She envisages bringing more phytomedicines to market through an innovative AI-based drug discovery engine and biopharmaceutical drug development program for disease-specific indications. Dr. Small-Howard has worked on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes.
More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ plant chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s Disease (PD), co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for plant-derived therapeutic compounds.
Dr. Small-Howard is the architect of a strategic vision at GB Sciences/GBS Global Biopharma to make safe, effective, standardized cannabinoid medicines available to patients where its use can be supported with rigorous evidence. To achieve standardization in their cannabinoid-containing, optimized therapeutic mixtures, the Company is using synthetic cannabinoids produced under current Good Manufacturing Practices (cGMP), which are identical in both structure and function to the homologous plant cannabinoids

Dr. Andrea L. Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Officer and Director of GB Sciences/GBS Global Biopharma, Inc.
Dr. Andrea Small-Howard has more than 20 years of scientific research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. Dr. Small-Howard has taken novel biological products from ideation through commercialization.
Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration (“US FDA”) and numerous international regulatory agencies. Currently, Dr. Andrea Small-Howard leverages her broad biopharmaceutical industry knowledge as the Chief Science Officer and member of the Board of Directors at both GB Sciences, Inc. (OTCQB:GBLX), and their wholly-owned Canadian subsidiary, GBS Global Biopharma, Inc. Dr. Small-Howard brings her passion for advancing research on phytochemical compounds to her current roles.
She envisages bringing more phytomedicines to market through an innovative AI-based drug discovery engine and biopharmaceutical drug development program for disease-specific indications. Dr. Small-Howard has worked on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes.
More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ plant chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s Disease (PD), co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for plant-derived therapeutic compounds.
Dr. Small-Howard is the architect of a strategic vision at GB Sciences/GBS Global Biopharma to make safe, effective, standardized cannabinoid medicines available to patients where its use can be supported with rigorous evidence. To achieve standardization in their cannabinoid-containing, optimized therapeutic mixtures, the Company is using synthetic cannabinoids produced under current Good Manufacturing Practices (cGMP), which are identical in both structure and function to the homologous plant cannabinoids

Dr. Andrea L. Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Officer and Director of GB Sciences/GBS Global Biopharma, Inc.
Dr. Andrea Small-Howard has more than 20 years of scientific research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. Dr. Small-Howard has taken novel biological products from ideation through commercialization.
Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration (“US FDA”) and numerous international regulatory agencies. Currently, Dr. Andrea Small-Howard leverages her broad biopharmaceutical industry knowledge as the Chief Science Officer and member of the Board of Directors at both GB Sciences, Inc. (OTCQB:GBLX), and their wholly-owned Canadian subsidiary, GBS Global Biopharma, Inc. Dr. Small-Howard brings her passion for advancing research on phytochemical compounds to her current roles.
She envisages bringing more phytomedicines to market through an innovative AI-based drug discovery engine and biopharmaceutical drug development program for disease-specific indications. Dr. Small-Howard has worked on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes.
More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ plant chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s Disease (PD), co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for plant-derived therapeutic compounds.
Dr. Small-Howard is the architect of a strategic vision at GB Sciences/GBS Global Biopharma to make safe, effective, standardized cannabinoid medicines available to patients where its use can be supported with rigorous evidence. To achieve standardization in their cannabinoid-containing, optimized therapeutic mixtures, the Company is using synthetic cannabinoids produced under current Good Manufacturing Practices (cGMP), which are identical in both structure and function to the homologous plant cannabinoids

Dr. Andrea L. Small-Howard
Chairman of the Scientific Advisory Board, Chief Science Officer and Director of GB Sciences/GBS Global Biopharma, Inc.
Dr. Andrea Small-Howard has more than 20 years of scientific research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. Dr. Small-Howard has taken novel biological products from ideation through commercialization.
Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration (“US FDA”) and numerous international regulatory agencies. Currently, Dr. Andrea Small-Howard leverages her broad biopharmaceutical industry knowledge as the Chief Science Officer and member of the Board of Directors at both GB Sciences, Inc. (OTCQB:GBLX), and their wholly-owned Canadian subsidiary, GBS Global Biopharma, Inc. Dr. Small-Howard brings her passion for advancing research on phytochemical compounds to her current roles.
She envisages bringing more phytomedicines to market through an innovative AI-based drug discovery engine and biopharmaceutical drug development program for disease-specific indications. Dr. Small-Howard has worked on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes.
More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ plant chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s Disease (PD), co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for plant-derived therapeutic compounds.
Dr. Small-Howard is the architect of a strategic vision at GB Sciences/GBS Global Biopharma to make safe, effective, standardized cannabinoid medicines available to patients where its use can be supported with rigorous evidence. To achieve standardization in their cannabinoid-containing, optimized therapeutic mixtures, the Company is using synthetic cannabinoids produced under current Good Manufacturing Practices (cGMP), which are identical in both structure and function to the homologous plant cannabinoids



